Our Gut-Brain Connection Revealed by “Organs-on-a-Chip” System
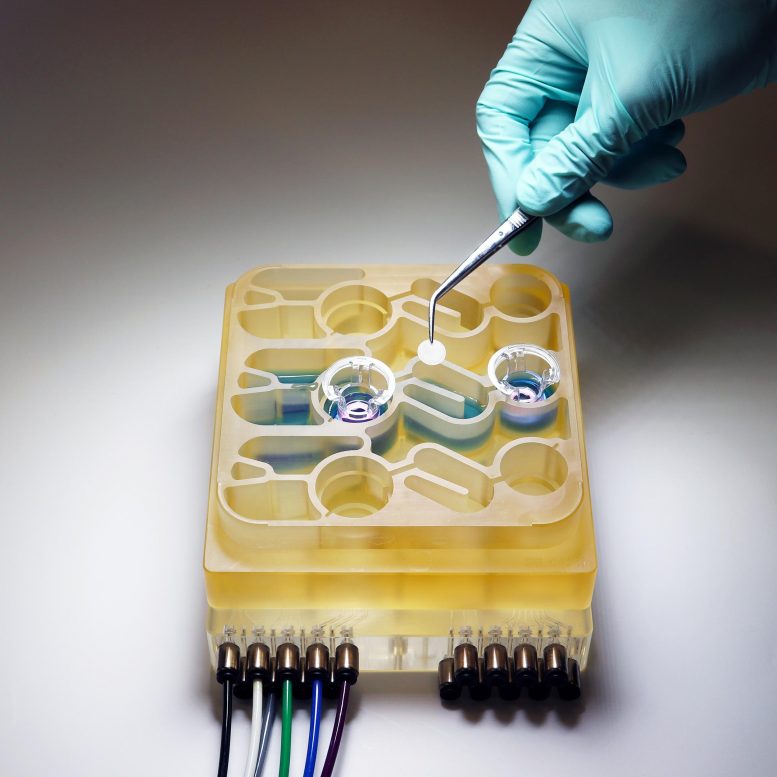
MIT researchers have developed an “organs-on-a-chip” system that replicates interactions between the brain, liver, and colon. Credit: Martin Trapecar, MIT
‘Organs-on-a-chip’ system sheds light on how bacteria in the human digestive tract may influence neurological diseases.
In many ways, our brain and our digestive tract are deeply connected. Feeling nervous may lead to physical pain in the stomach, while hunger signals from the gut make us feel irritable. Recent studies have even suggested that the bacteria living in our gut can influence some neurological diseases.
Modeling these complex interactions in animals such as mice is difficult to do, because their physiology is very different from humans’. To help researchers better understand the gut-brain axis, MIT researchers have developed an “organs-on-a-chip” system that replicates interactions between the brain, liver, and colon.
Using that system, the researchers were able to model the influence that microbes living in the gut have on both healthy brain tissue and tissue samples derived from patients with Parkinson’s disease. They found that short-chain fatty acids, which are produced by microbes in the gut and are transported to the brain, can have very different effects on healthy and diseased brain cells.
“While short-chain fatty acids are largely beneficial to human health, we observed that under certain conditions they can further exacerbate certain brain pathologies, such as protein misfolding and neuronal death, related to Parkinson’s disease,” says Martin Trapecar, an MIT postdoc and the lead author of the study.
Linda Griffith, the School of Engineering Professor of Teaching Innovation and a professor of biological engineering and mechanical engineering, and Rudolf Jaenisch, an MIT professor of biology and a member of MIT’s Whitehead Institute for Medical Research, are the senior authors of the paper, which appears today (January 29, 2021) in Science Advances.
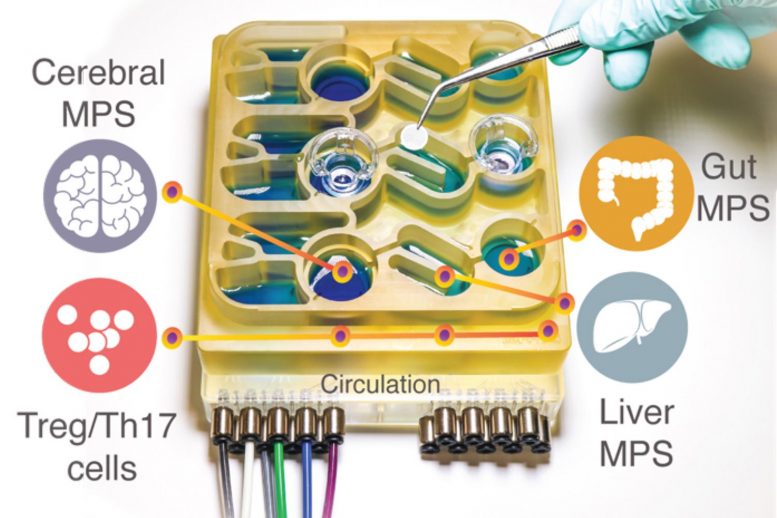
MIT researchers have developed an “organs-on-a-chip” system that replicates interactions between the brain, liver, and colon. Credit: Martin Trapecar, MIT
The gut-brain connection
For several years, Griffith’s lab has been developing microphysiological systems — small devices that can be used to grow engineered tissue models of different organs, connected by microfluidic channels. In some cases, these models can offer more accurate information on human disease than animal models can, Griffith says.
In a paper published last year, Griffith and Trapecar used a microphysiological system to model interactions between the liver and the colon. In that study, they found that short-chain fatty acids (SCFAs), molecules produced by microbes in the gut, can worsen autoimmune inflammation associated with ulcerative colitis under certain conditions. SCFAs, which include butyrate, propionate, and acetate, can also have beneficial effects on tissues, including increased immune tolerance, and they account for about 10 percent of the energy that we get from food.
In the new study, the MIT team decided to add the brain and circulating immune cells to their multiorgan system. The brain has many interactions with the digestive tract, which can occur via the enteric nervous system or through the circulation of immune cells, nutrients, and hormones between organs.
Several years ago, Sarkis Mazmanian, a professor of microbiology at Caltech, discovered a connection between SCFAs and Parkinson’s disease in mice. He showed that SCFAs, which are produced by bacteria as they consume undigested fiber in the gut, sped up the progression of the disease, while mice raised in a germ-free environment were slower to develop the disease.
Griffith and Trapecar decided to further explore Mazmanian’s findings, using their microphysiological model. To do that, they teamed up with Jaenisch’s lab at the Whitehead Institute. Jaenisch had previously developed a way to transform fibroblast cells from Parkinson’s patients into pluripotent stem cells, which can then be induced to differentiate into different types of brain cells — neurons, astrocytes, and microglia.
More than 80 percent of Parkinson’s cases cannot be linked to a specific gene mutation, but the rest do have a genetic cause. The cells that the MIT researchers used for their Parkinson’s model carry a mutation that causes accumulation of a protein called alpha synuclein, which damages neurons and causes inflammation in brain cells. Jaenisch’s lab has also generated brain cells that have this mutation corrected but are otherwise genetically identical and from the same patient as the diseased cells.
Griffith and Trapecar first studied these two sets of brain cells in microphysiological systems that were not connected to any other tissues, and found that the Parkinson’s cells showed more inflammation than the healthy, corrected cells. The Parkinson’s cells also had impairments in their ability to metabolize lipids and cholesterol.
Opposite effects
The researchers then connected the brain cells to tissue models of the colon and liver, using channels that allow immune cells and nutrients, including SCFAs, to flow between them. They found that for healthy brain cells, being exposed to SCFAs is beneficial, and helps them to mature. However, when brain cells derived from Parkinson’s patients were exposed to SCFAs, the beneficial effects disappeared. Instead, the cells experienced higher levels of protein misfolding and cell death.
These effects were seen even when immune cells were removed from the system, leading the researchers to hypothesize that the effects are mediated by changes to lipid metabolism.
“It seems that short-chain fatty acids can be linked to neurodegenerative diseases by affecting lipid metabolism rather than directly affecting a certain immune cell population,” Trapecar says. “Now the goal for us is to try to understand this.”
The researchers also plan to model other types of neurological diseases that may be influenced by the gut microbiome. The findings offer support for the idea that human tissue models could yield information that animal models cannot, Griffith says. She is now working on a new version of the model that will include micro blood vessels connecting different tissue types, allowing researchers to study how blood flow between tissues influences them.
“We should be really pushing development of these, because it is important to start bringing more human features into our models,” Griffith says. “We have been able to start getting insights into the human condition that are hard to get from mice.”
Reference: 29 January 2021, Science Advances.
The research was funded by DARPA, the National Institutes of Health, the National Institute of Biomedical Imaging and Bioengineering, the National Institute of Environmental Health Sciences, the Koch Institute Support (core) Grant from the National Cancer Institute, and the Army Research Office Institute for Collaborative Biotechnologies.
